Where in the Cell Does Fatty Acid Synthesis Occur
In biochemistry, fatty acid synthesis is the founding of fatty acids from acetyl group-CoA and NADPH through and through the sue of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into sebaceous acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway likewise provides the glycerol with which triad fatty acids can combine (away agency of ester bonds) to form triglycerides (likewise known as "triacylglycerols" – to distinguish them from fatty "acids" – OR simply every bit "fat"), the final mathematical product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol grouping is phosphorylated with a group such arsenic phosphatidylcholine, a phospholipid is funnel-shaped. Phospholipids variety the bulge of the lipide bilayers that make up cell membranes and surrounds the organelles within the cells (e.g. the cell karyon, mitochondria, endoplasmic reticulum, Golgi setup etc.).
Straight-chain oleaginous acids [edit out]
Straight-chain fatty acids occur in two types: soppy and unsaturated.
Saturated straight-range fatty acids [edit]

Synthesis of saturated superfatted acids via fatty acid synthase II in Escherichia coli
Much ilk β-oxidation, straight-chain fatty acid deductive reasoning occurs via the sixer recurring reactions shown below, until the 16-carbon palmitic acid is produced.[1] [2]
The diagrams presented show how fatty acids are synthesized in microorganisms and list the enzymes found in E. coli.[1] These reactions are performed by fatty acid synthase Cardinal (FASII), which in general carry tenfold enzymes that move A unmatchable knotty. FASII is present in prokaryotes, plants, fungi, and parasites, every bit well as in mitochondria.[3]
In animals, as cured as some fungi such as yeast, these same reactions occur on fatty acid synthase I (FASI), a thumping dimeric protein that has all of the enzymatic activities required to produce a fatty battery-acid. FASI is less efficient than FASII; however, IT allows for the formation of more molecules, including "medium-chain" fatty acids via early chain termination.[3]
Once a 16:0 carbon fatty virulent has been shaped, IT backside get a number of modifications, resulting in desaturation and/operating theater elongation. Elongation, starting with stearate (18:0), is performed principally in the ER by several membrane-bound enzymes. The enzymatic steps committed in the elongation process are principally the same as those carried retired by FAS, but the four principal successive steps of the extension are performed by individual proteins, which whitethorn be physically associated.[4] [5]
| Step | Enzyme | Reaction | Description |
|---|---|---|---|
| (a) | Acetyl CoA:ACP transacylase | | Activates acetyl CoA for reaction with malonyl-ACP |
| (b) | Malonyl CoA:ACP transacylase | | Activates malonyl CoA for chemical reaction with acetyl-ACP |
| (c) | 3-ketoacyl-ACP synthase | | Reacts ACP-bound acyl group chain with chemical chain-extending malonyl-ACP |
| (d) | 3-ketoacyl-ACP reductase | | Reduces the carbon 3 ketone to a hydroxyl radical group |
| (e) | 3-Hydroxyacyl ACP dehydrase | | Eliminates water |
| (f) | Enoyl-ACP reductase | | Reduces the C2-C3 double bond. |
| Abbreviations: ACP – Acyl carrier protein, CoA – Coenzyme A, NADP – Nicotinamide adenine dinucleotide phosphate. | |||
Take note that during oily synthesis the reduction agent is NADPH, whereas NAD is the oxidizing agent in beta-oxidation (the breakdown of fat acids to acetyl-CoA). This difference exemplifies a world-wide rule that NADPH is consumed during biosynthetic reactions, whereas NADH is generated in Energy-yielding reactions.[6] (Thus NADPH is also required for the synthesis of cholesterol from acetyl-CoA; while NADH is generated during glycolysis.) The generator of the NADPH is two-fold. When malate is oxidatively decarboxylated by "NADP+-linked malic enzyme" to form pyruvate, CO2 and NADPH are planned. NADPH is also formed by the pentose phosphate tract which converts glucose into ribose, which can atomic number 4 used in synthesis of nucleotides and nucleic acids, or IT can be catabolized to pyruvate.[6]
Conversion of carbohydrates into fatty acids [edit out]
In humans, fatty acids are spider-shaped from carbohydrates preponderantly in the liver and adipose tissue, as intimately as in the mammary glands during lactation.
The pyruvate produced by glycolysis is an important intermediary in the conversion of carbohydrates into fatty acids and cholesterol.[6] This occurs via the changeover of pyruvate into acetyl-CoA in the mitochondrion. However, this ethanoyl radica CoA needs to be transported into cytosol where the deduction of fatty acids and cholesterol occurs. This cannot fall out directly. To obtain cytosolic ethanoyl group-CoA, citrate (produced by the condensation of acetyl CoA with oxalacetate) is removed from the citric acid oscillation and carried across the inner mitochondrial membrane into the cytosol.[6] There it is cleaved by ATP citrate lyase into ethanoyl radica-CoA and oxaloacetate. The oxaloacetate can be used for gluconeogenesis (in the coloured), or it fire be returned into chondriosome as malate.[7] The cytosolic acetyl-CoA is carboxylated by acetyl CoA carboxylase into malonyl CoA, the first committed step in the synthesis of fatty acids.[7] [8]
Animals cannot resynthesize carbohydrates from fatty acids [edit]
The main fuel stored in the bodies of animals is fat. A young adult human's juicy stores modal between about 15– 20 kilogram, but varies greatly depending on long time, gender, and individual disposition.[9] In line, the human body stores only about 400 g of glycogen, of which 300 g is locked inside the system muscles and is out of stock to the body as a gross. The 100 g or so of animal starch stored in the colored is insufficient within one and only day of starvation.[10] Thereafter the glucose that is released into the blood by the colorful for general utilize aside the body tissues, has to be synthesized from the glucogenic amino acids and a few other gluconeogenic substrates, which serve not let in roly-poly acids.[11]
Butterball acids are broken down to ethanoyl group-CoA by means of important oxidization inside the mitochondria, whereas fatty acids are synthesized from acetyl-CoA outside the mitochondrion, in the cytosol. The two pathways are distinct, not only in where they pass, but also in the reactions that occur, and the substrates that are used. The two pathways are reciprocally inhibitory, preventing the acetyl group-CoA produced by beta-oxidation from entering the logical tract via the acetyl-CoA carboxylase reaction.[11] Information technology can also not be converted to pyruvate every bit the pyruvate decarboxylation reaction is irreversible.[10] Instead it condenses with oxaloacetate, to enter the citric acid cycle. During each turn of the cycle, two carbon atoms leave the cycle as CO2 in the decarboxylation reactions catalyzed by isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase. Thus each turn of the citric acid cycle oxidizes an acetyl-CoA unit piece regenerating the oxalacetate corpuscle with which the ethanoyl group-CoA had originally combined to form citric acid. The decarboxylation reactions occur before malate is formed in the oscillation. Malate is the only substance that can be removed from the mitochondrion to enter the gluconeogenic tract to form glucose or glycogen in the colorful or whatever other weave.[11] There tooshie therefore personify no net conversion of fatty acids into glucose.
Only plants possess the enzymes to convert acetyl-CoA into oxaloacetate from which malate can equal precast to at last personify born-again to glucose.[11]
Regulation
Acetyl-CoA is tassel-shaped into malonyl-CoA by acetyl-CoA carboxylase, at which point malonyl-CoA is destined to give into the fatty acid synthetic thinking tract. Acetyl-CoA carboxylase is the pointedness of rule in saturated straight-chain oily dose synthesis, and is subject to both phosphorylation and allosteric regulation. Regulation by phosphorylation occurs mostly in mammals, while allosteric regulation occurs in well-nig organisms. Allosteric contain occurs A feedback forbiddance by palmitoyl-CoA and activation by citrate. When there are high levels of palmitoyl-CoA, the final product of saturated fatty acerb synthesis, it allosterically inactivates acetyl-CoA carboxylase to prevent a bod-up of fatty acids in cells. Citrate acts to activate ethanoyl radica-CoA carboxylase under high levels, because high levels indicate that there is plenty acetyl-CoA to feed into the Sir Hans Adolf Krebs cycle and conserve energy.[12]
Inebriated plasma levels of insulin in the parentage plasma (e.g. after meals) cause the dephosphorylation of ethanoyl group-CoA carboxylase, thusly promoting the formation of malonyl-CoA from acetyl-CoA, and consequently the conversion of carbohydrates into fatty acids, piece epinephrine and glucagon (released into the blood during starvation and exercise) effort the phosphorylation of this enzyme, inhibiting lipogenesis in favou fatty unpleasant oxidation via beta-oxidisation.[6] [8]
Monounsaturated transparent chain fatty acids [edit]
Anaerobic desaturation [edit]
Many bacterium use the anaerobiotic pathway for synthesizing polyunsaturated fatty acids. This pathway does not utilize oxygen and is hanging down on enzymes to insert the doubly bond before elongation utilizing the convention fatty acid deductive reasoning machinery. In Escherichia coli, this pathway is well understood.

Synthesis of unsaturated fatty acids via anaerobic desaturation
- FabA is a β-hydroxydecanoyl-ACP dehydrase – it is specialised for the 10-carbon saturated fatty acid synthesis liaise (β-hydroxydecanoyl-ACP).
- FabA catalyzes the dehydration of β-hydroxydecanoyl-ACP, causation the release of water and insertion of the double bond between C7 and C8 reckoning from the methyl end. This creates the trans-2-decenoyl intermediate.
- Either the trans-2-decenoyl intermediate can follow shunted to the modal saturated fatty acid synthesis pathway away FabB, where the double bond will be hydrolyzed and the ultimate ware will be a saturated fatty acid, or FabA volition catalyse the isomerisation into the cis-3-decenoyl intermediate.
- FabB is a β-ketoacyl-ACP synthase that elongates and channels intermediates into the mainstream fatty acid synthesis pathway. When FabB reacts with the Commonwealth of Independent States-decenoyl intermediate, the final product after elongation will be an unsaturated fatty acid.[13]
- The 2 main unsaturated fatty acids ready-made are Palmitoleoyl-ACP (16:1ω7) and cis-vaccenoyl-ACP (18:1ω7).[14]
Most bacterium that undergo anaerobic desaturation contain homologues of FabA and FabB.[15] Clostridium are the main exception; they have a fresh enzyme, yet to live known, that catalyzes the establishment of the cis two-fold bond.[14]
Regulation
This pathway undergoes transcriptional regulation by FadR and FabR. FadR is the more extensively studied protein and has been attributed bifunctional characteristics. Information technology Acts of the Apostles American Samoa an activator of fabA and fabB transcription and as a repressor for the β-oxidation regulon. In contrast, FabR acts as a repressor for the transcription of fabA and fabB.[13]
Aerophilic desaturation [edit]
Oxidative desaturation is the most widespread footpath for the synthesis of unsaturated fatty acids. Information technology is utilized in all eukaryotes and close to prokaryotes. This pathway utilizes desaturases to synthesize unsaturated fatty acids from full-duration saturated fatty acid substrates.[16] All desaturases require oxygen and ultimately take in NADH even though desaturation is an aerobic process. Desaturases are specific for the large bond they induce in the substrate. In Hay bacillus, the desaturase, Δ5-Des, is specific for inducing a CIS-double bond at the Δ5 position.[7] [16] Saccharomyces cerevisiae contains one desaturase, Ole1p, which induces the cis-double bond at Δ9.[7]
In mammals the aerobic desaturation is catalyzed away a complex of three tissue layer-bound enzymes (NADH-cytochrome b5 reductase, cytochrome b5 , and a desaturase). These enzymes admit molecular oxygen, O2, to interact with the sopping fatty acyl-CoA string, forming a double bond and two molecules of irrigate, H2O. Two electrons get along from NADH + H+ and two from the widowed bond in the greasy acid chain.[6] These mammal enzymes are, however, incapable of introducing double bonds at carbon atoms beyond C-9 in the fatty acid chain.[N.B. 1].) Hence mammals cannot synthesise linoleate or linolenate (which have double bonds at the C-12 (= Δ12), or the C-12 and C-15 (= Δ12 and Δ15) positions, respectively, Eastern Samoa well as at the Δ9 position), nor the polyunsaturated, 20-carbon arachidonic acid that is derived from linoleate. These are whol termed requisite fatty acids, meaning that they are required aside the organism, but can only be supplied via the diet. (Arachidonic acidulous is the precursor the prostaglandins which fulfi a panoramic multifariousness of functions as local anesthetic hormones.)[6]
Odd-chain superfatted acids [edit]
Unusual-chain suety acids (OCFAs) are those oleaginous acids that contain an odd number of carbon atoms. The most common OCFAs are the vivid C15 and C17 derivatives, respectively pentadecanoic battery-acid and heptadecanoic blistering.[17] The deductive reasoning of even-chained fatty acid synthesis is done by collection acetyl group-CoA precursors, however, propionyl-CoA or else of acetyl-CoA is used as the primer for the biosynthesis of long-chain fatty acids with an odd number of carbon atoms.[18]
Regulation In B. subtilis, this tract is regulated past a two-component system: DesK and DesR. DesK is a membrane-associated kinase and DesR is a transcriptional governor of the stilbestrol gene.[7] [16] The regulation responds to temperature; when in that location is a drop by temperature, this cistron is upregulated. Unsaturated oily acids increase the fluidity of the tissue layer and steady it under lower temperatures. DesK is the sensor protein that, when there is a decrease in temperature, will autophosphorylate. DesK-P bequeath transfer its phosphoryl aggroup to DesR. Ii DesR-P proteins will dimerize and bind to the DNA promoters of the des factor and levy RNA polymerase to begin transcription.[7] [16]
Pseudomonas aeruginosa
Generally, both anaerobiotic and aerobic dull fatty window pane synthesis will non occur inside the same organization, however Pseudomonas aeruginosa and Vibrio ABE-1 are exceptions.[19] [20] [21] While P. aeruginosa undergoes primarily anaerobiotic desaturation, it also undergoes two aerobic pathways. One pathway utilizes a Δ9-desaturase (DesA) that catalyzes a double bond formation in membrane lipids. Another pathway uses two proteins, DesC and DesB, unitedly to play a Δ9-desaturase, which inserts a double bond into a saturated fat acid-CoA molecule. This second pathway is regulated by represser protein DesT. DesT is besides a repressor of fabAB expression for anaerobic desaturation when in presence of exogenous unsaturated adipose acids. This functions to coordinate the expression of the ii pathways within the organism.[20] [22]
Ramous-concatenation fatty acids [edit]
Biramous chain fatty acids are usually saturated and are found in two different families: the iso-serial publication and anteiso-series. It has been found that Actinomycetales arrest unique ramify-chain fatty acid synthesis mechanisms, including that which forms tuberculosteric superman.
Furcate-chain greasy acid synthesizing arrangement [edit out]

Valine priming

Leucine primer
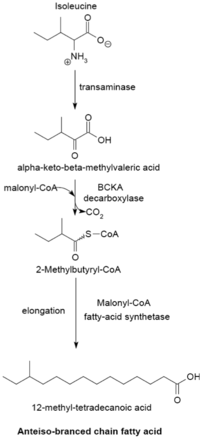
Isoleucine primer
Synthetic pathways of the forficate-chain buttery acid synthesizing system given differing primers
The branched-mountain chain fatty acid synthesizing system uses α-keto acids as primers. This system is distinct from the divided-chain fatty acid synthetase that utilizes short-chain acyl group-CoA esters As primers.[23] α-Keto acid primers are derived from the transamination and decarboxylation of valine, leucine, and isoleucine to form 2-methylpropanyl-CoA, 3-methylbutyryl-CoA, and 2-Methylbutyryl-CoA, severally.[24] 2-Methylpropanyl-CoA primers derived from valine are elongated to produce even-numbered iso-series fatty acids such as 14-methyl-pentadecanoic (isopalmitic) acid, and 3-methylbutyryl-CoA primers from leucine may be put-upon to form odd-numbered iso-series fatty acids so much as 13-methyl radical-myristic acid. 2-Methylbutyryl-CoA primers from isoleucine are elongated to variant anteiso-series fatty acids containing an odd number of carbon paper atoms such as 12-Methyl myristic acid.[25] Decarboxylation of the fuze precursors occurs through and through the branched-chain α-keto acid decarboxylase (BCKA) enzyme. Elongation of the fatty acid follows the same biosynthetic pathway in Escherichia coli accustomed produce straight-chain fatty acids where malonyl-CoA is used as a chain extender.[26] The major close products are 12–17 carbon branched-chain fatty acids and their composition tends to be uniform and characteristic for many bacterial species.[25]
BCKA decarboxylase and congeneric activities of α-keto acid substrates
The BCKA decarboxylase enzyme is composed of two subunits in a tetrameric structure (A2B2) and is essential for the synthesis of branched-chain roly-poly acids. IT is responsible for the decarboxylation of α-keto acids formed by the transamination of valine, leucine, and isoleucine and produces the primers used for branched-chain fatty acid synthesis. The activity of this enzyme is much higher with branched-chain α-keto acid substrates than with straight-Ernst Boris Chain substrates, and in Bacillus species its specificity is highest for the isoleucine-plagiarised α-keto-β-methylvaleric acid, followed by α-ketoisocaproate and α-ketoisovalerate.[25] [26] The enzyme's high affinity toward branched-Sir Ernst Boris Chain α-keto acids allows IT to function as the primer donating organisation for branched-chain fatty acid synthetase.[26]
| Substrate | BCKA activity | Atomic number 272 Produced (nmol/min mg) | Km (μM) | Vmax (nmol/Min dialect mg) |
|---|---|---|---|---|
| L-α-keto-β-methyl-valerate | 100% | 19.7 | <1 | 17.8 |
| α-Ketoisovalerate | 63% | 12.4 | <1 | 13.3 |
| α-Ketoisocaproate | 38% | 7.4 | <1 | 5.6 |
| Pyruvate | 25% | 4.9 | 51.1 | 15.2 |
Factors affecting chain duration and pattern distribution
α-Keto acid primers are accustomed produce forficate-chain fatty acids that, generally, are 'tween 12 and 17 carbons in length. The proportions of these branched-chemical chain fat acids tend to be uniform and consistent among a particular bacterial species but may be altered due to changes in malonyl-CoA concentration, temperature, or ignite-stable factors (HSF) present.[25] All of these factors Crataegus oxycantha dissemble chain length, and HSFs have been demonstrated to alter the specificity of BCKA decarboxylase for a finicky α-keto acid substrate, thus shifting the ratio of branched-chain fatty acids produced.[25] An increase in malonyl-CoA engrossment has been shown to result in a larger balance of C17 fatty acids produced, up until the optimum concentration (≈20μM) of malonyl-CoA is reached. Decreased temperatures also be given to shift the greasy-acid distribution slightly toward C17 fatty-acids in Bacillus species.[23] [25]
Branch-strand fatty acid synthase [edit]
This system functions similarly to the branch-Ernst Boris Chain fatty acid synthesizing organisation, however it uses short-chain carboxylic acids arsenic primers rather of of import-keto acids. Generally, this method acting is put-upon by bacteria that do not have the ability to perform the branch-chain fatty acid system using alpha-keto primers. Typical short-chain primers let in isovalerate, isobutyrate, and 2-methyl butyrate. In generalised, the acids required for these primers are taken up from the environment; this is often seen in ruminal bacteria.[27]
The overall reaction is:
- Isobutyryl-CoA + 6 malonyl-CoA +12 NADPH + 12H+ → Isopalmitic acid + 6 CO2 12 NADP + 5 H2O + 7 CoA[23]
The difference between (straight-chain) fatty acid synthase and arm-chain fatty acid synthase is substratum specificity of the enzyme that catalyzes the reaction of acyl-CoA to acyl group-ACP.[23]
Omega-alicyclic fatty acids [edit]

Omega-alicyclic fatty acids typically contain an Z-terminal propyl or butyryl cyclic chemical group and are some of the major membrane fatty acids found in several species of bacteria. The fatty acid synthetase used to produce omega-alicyclic fatty acids is too used to produce tissue layer branched-chain fatty acids. In bacteria with membranes composed mainly of omega-alicyclic fatty acids, the ply of cyclic carboxylic acid-CoA esters is a good deal greater than that of branched-chain primers.[23] The synthesis of heterocyclic primers is non well understood but it has been suggested that chemical mechanism involves the conversion of sugars to shikimic acid which is then converted to cyclohexylcarboxylic back breaker-CoA esters that serve American Samoa primers for omega-alicyclic fat acid deductive reasoning[27]
Tuberculostearic acid synthesis [blue-pencil]
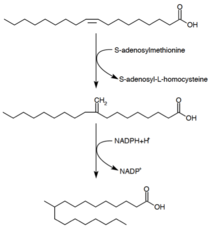
Mechanics of the synthesis of tuberculostearic acid
Tuberculostearic acid (D-10-Methylstearic acid) is a pure fatty acid that is known to be produced by Mycobacterium spp. and two species of Streptomyces. It is paddle-shaped from the precursor oleic virulent (a unsaturated fatty acid).[28] Subsequently oleic acid is esterified to a phospholipid, S-adenosyl-methionine donates a methyl group group to the stunt woman bond of oleic acid.[29] This methylation reaction forms the mediate 10-methylene-octadecanoyal. Successive reduction of the residue, with NADPH as a cofactor, results in 10-methylstearic caustic[24]
See too [redact]
- Fatso acid
- Essential fatty acidulous
- Fatty acid metabolism
- Fatty acid synthase
Footnote [edit]
- ^ The position of the atomic number 6 atoms in a fatty acrid bum be indicated from the COOH- (operating theater carboxy) end, or from the -CH3 (or methyl radical) end. If indicated from the -COOH remainder, then the C-1, C-2, C-3, ... .(etc.) notation is used (risque numerals in the plot on the right, where C-1 is the –COOH carbon). If the position is counted from the other, -CH3, oddment then the position is indicated by the ω-n note (numerals in red, where ω-1 refers to the methyl carbon copy).

Numbering of carbon atoms
The positions of the double bonds in a buttery acid Sir Ernst Boris Chain can, therefore, be indicated in two ways, using the C-n or the ω-n notation. Thus, in an 18 carbon fatty acid, a double bond between C-12 (or ω-7) and C-13 (or ω-6) is reported either as Δ12 if counted from the –COOH end (indicating but the "rootage" of the large tie), surgery as ω-6 (or omega-6 fatty acid) if counting from the -CH3 oddment. The "Δ" is the Greek letter "delta", which translates into "D" (for Double bond) in the Roman alphabet. Omega (ω) is the last letter in the Greek alphabet, and is therefore in use to indicate the "last" carbon atom in the fatty acid chain. Since the ω-n annotation is victimised virtually exclusively to point the positions of the forked bonds enveloping to the -CH3 end in essential fatty acids, there is no necessity for an equivalent "Δ"-like notation – the use of the "ω-n" notation always refers to the position of a double bond.
References [edit]
- ^ a b Dijkstra, Albert J., R. J. Hamilton, and Wolf Hamm. "Fatty Acid Biosynthesis." Trans Fatty Acids. Oxford: Blackwell Pub., 2008. 12. Photographic print.
- ^ "MetaCyc pathway: superpathway of fatty acids biosynthesis (E. coli)".
- ^ a b "Fatty Acids: Straight-chain Saturated, Structure, Occurrence and Biosynthesis." Lipid Library – Lipoid Alchemy, Biology, Engineering and Analysis. Web. 30 Apr. 2011. <http://lipidlibrary.aocs.org/lipids/fa_sat/index.htm Archived 21 July 2011 at the Wayback Machine>.
- ^ "MetaCyc pathway: stearate biosynthesis I (animals)".
- ^ "MetaCyc pathway: identical long-chain molecule superfatted acid biosynthesis II".
- ^ a b c d e f g Stryer, Lubert (1995). Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 559–565, 614–623. ISBN0-7167-2009-4.
- ^ a b c d e f Ferre, P.; F. Foufelle (2007). "SREBP-1c Recording Factor and Lipid Homeostasis: Objective Perspective". Internal secretion Enquiry. 68 (2): 72–82. Interior Department:10.1159/000100426. PMID 17344645. Retrieved 30 August 2010.
this process is outlined graphically in page 73
- ^ a b Voet, Donald; Judith G. Voet; Charlotte W. Pratt (2006). Fundamentals of Biochemistry, 2nd Variation . John the Evangelist Wiley and Sons, INC. pp. 547, 556. ISBN0-471-21495-7.
- ^ Sloan, A.W; Koeslag, J.H.; Bredell, G.A.G. (1973). "Body composition forg capacity and work efficiency of participating and inactive young hands". European Journal of Applied Physiology. 32: 17–24. doi:10.1007/bf00422426. S2CID 39812342.
- ^ a b Stryer, Lubert (1995). Biochemistry (Fourth male erecticle dysfunction.). New York: W.H. Freeman and Company. pp. 581–602, 613, 775–778. ISBN0-7167-2009-4.
- ^ a b c d Stryer, Lubert (1995). "Fat acid metabolic process.". In: Biochemistry (Quarter ed.). New York: W.H. Freewoman and Company. pp. 603–628. ISBN0-7167-2009-4.
- ^ Diwan, Joyce J. "Fatty Acid Synthesis." Rensselaer Polytechnic Institute (RPI) :: Architecture, Business, Engineering, IT, Humanities, Scientific discipline. Web. 30 Apr. 2011. <http://rpi.edu/dept/bcbp/molbiochem/MBWeb/mb2/part1/fasynthesis.htm Archived 7 June 2011 at the Wayback Machine>.
- ^ a b Feng, Youjun, and John ECronan. "Complex binding of the FabR repressor of bacterial unsaturated fat acid biosynthesis to its cognate promoters." Building block microbiology 80.1 (2011):195–218.
- ^ a b Zhu, Lei, et alii. "Functions of the Clostridia acetobutylicium FabF and FabZ proteins in unsaturated fatty acid biogenesis." BMC Microbiology 9(2009):119.
- ^ Wang, Haihong, and John ECronan. "Functional replacement of the FabA and FabB proteins of Escherichia coli suety acid synthesis aside Enterococcus faecalis FabZ and FabF homologues." Journal of Biological Chemistry 279.33 (2004):34489-95.
- ^ a b c d Mansilla, Mara C, and Diegode Mendoza. "The Bacillus globigii desaturase: a model to understand phospholipid adjustment and temperature sensing." Archives of Microbiology 183.4 (2005):229-35.
- ^ Pfeuffer, Calophyllum longifolium; Jaudszus, Anke (2016). "Pentadecanoic and Heptadecanoic Acids: Many-sided Left over-Chain of mountains Fatty Acids". Advances in Nourishment. 7 (4): 730–734. doi:10.3945/an.115.011387. PMC4942867. PMID 27422507.
- ^ David Roland Smith, S. (1994). "The Perch-like Fatty Acid Synthase: One Gene, One Polypeptide, Seven Enzymes". The FASEB Journal. 8 (15): 1248–1259. Department of the Interior:10.1096/fasebj.8.15.8001737. PMID 8001737. S2CID 22853095.
- ^ Wada, M, N. Fukunaga, and S. Sasaki. "Mechanism of biosynthesis of monounsaturated fatty acids in Pseudomonas sp. breed E-3, a psychrotrophic bacteria." Journal of Bacteriology 171.8 (1989):4267-71.
- ^ a b Subramanian, Chitra, Charles ORock, and Yong-MeiZhang. "DesT coordinates the expression of anaerobic and aerophilic pathways for monounsaturated fatty acid biosynthesis in Pseudomonas aeruginosa." Journal of Bacteriology 192.1 (2010):280-5.
- ^ Morita, N, et al. "Both the anaerobiotic pathway and oxidative desaturation are involved in the synthetic thinking of unsaturated oleaginous acids in Vibrio sp. strain ABE-1." FEBS Letters 297.1–2 (1992):9–12.
- ^ Zhu, Kun, et al. "Two aerobiotic pathways for the formation of unsaturated fat person acids in Pseudomonas aeruginosa." Molecular microbiology 60.2 (2006):260-73.
- ^ a b c d e Kaneda, Toshi. "Iso- and Anteiso-Sebaceous Acids in Bacterium: Biogenesis, Function, and Taxonomic Implication." Microbiological Reviews 55.2 (1991): 288–302
- ^ a b "Forked-chain Butterball Acids, Phytanic Acidulent, Tuberculostearic Acid Iso/anteiso- Fatty Acids." Lipide Library – Lipid Interpersonal chemistry, Biota, Technology and Analysis. Web. 1 Crataegus oxycantha 2011. "Archived copy". Archived from the original on 12 January 2010. Retrieved 8 March 2014. CS1 maint: archived copy as title (link up).
- ^ a b c d e f Naik, Devaray N., and Toshi Kaneda. "Biosynthesis of Branched Long-chain Fatty Acids by Species of B: Relative Activity of Ternary α-keto Loony toons Substrates and Factors Affecting Chain Length." Can. J. Microbiol. 20 (1974): 1701–708.
- ^ a b c Oku, Hirosuke, and Toshi Kaneda. "Biosynthesis of Branched-chain Fatty Acids in Bacillis Subtilis." The Journal of Biological Chemistry 263.34 (1988): 18386-8396.
- ^ a b Christie, William W. "Fatty Acids: Natural Alicyclic Structures, Occurrence, and Biochemistry." The AOCS Lipid Library. 5 Apr. 2011. Web. 24 Apr. 2011. <"Archived copy" (PDF). Archived from the original (PDF) on 21 July 2011. Retrieved 2 May 2011. CS1 maint: archived simulate as title (link)>.
- ^ Ratledge, Colin, and John Stanford. The Biology of the Mycobacteria. London: Academic, 1982. Print.
- ^ Kubica, George P., and Lawrence G. Wayne. The Mycobacteria: a Sourcebook. New York: Thomas Dekker, 1984. Print.
External links [blue-pencil]
- Overview at Rensselaer Polytechnic Institute
- Overview at Indiana State University
Where in the Cell Does Fatty Acid Synthesis Occur
Source: https://en.wikipedia.org/wiki/Fatty_acid_synthesis
0 Response to "Where in the Cell Does Fatty Acid Synthesis Occur"
Enregistrer un commentaire